Biofilm – a brief introduction
Biofilm, a brief introduction
Biofilms and their medical importance
Biofilms are clusters of one or more living microorganisms, or microbes, such as bacteria, fungi, and viruses that are attached to a surface and embedded in a self-produced matrix designed for the survival of organisms.
And why should we pay attention to biofilms?
From a medical perspective, biofilms have significant effects on human health and medicine. Bacterial biofilm is a key reason for the contamination of medical devices and the generation of microbial and chronic infections in the body. In fact, biofilms are the source of a number of human diseases as they cause serious infections and have antimicrobial drug resistant features. Especially, the microbial cells living inside biofilms are less likely to be affected by to antibiotics and disinfectants, making biofilm infections very difficult to treat.
For these reasons, knowledge on biofilms, their prevention, and treatment can enhance medical and dental practices.
What are biofilms?
Biofilms represent a survival mechanism of microorganisms and are therefore ubiquitous in nature. They are complex, slime-encased communities of microbes which are often seen as slime layers on objects in water or at water-air interfaces [1, 2]. Especially, microbes in aquatic environments are more often found in the sessile form (in the so called biofilms on surfaces) than in the planktonic, free-floating, form [1, 2].
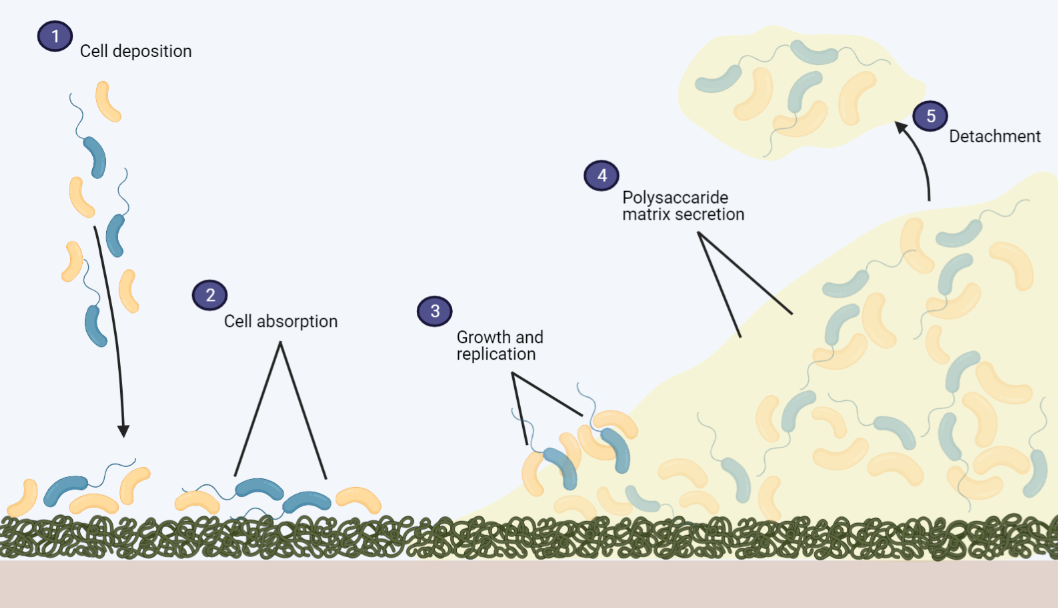
 How do biofilms form?
How do biofilms form?
The ability of microbes to form biofilms is ancient and has been detected back to 3.4 billion years ago . Biofilm formation can occur at any given surface if the environment contains protein and other molecules. The formation initiates with the microbes attaching to the conditioned surface [2, 3]. Gradually, the microbes form a slimy matrix of various polymers which is called an extracellular matrix (ECM) or an extracellular polymeric substance (EPS). This matrix includes proteins, polysaccharides, glycolipids, glycoproteins, and DNA. The polymer combination in the EPS and ECM depends on the microbes in the biofilm, and the attachment to the object is stabled by the EPS . Over time, the biofilm thickens and matures together with the reproduction of the microbes and secretion of additional polymers. Eventually, the location and condition in the biofilm becomes harmful to the microbes, and this promotes detachment and escape of the cells [1, 2].
Inside this complex and dynamic community of microorganisms in a mature biofilm, the microbes interact in a variety of ways. For instance, by benefitting from each other’s waste products or the transfer of genetic material in the EPS between cells or species which promotes functional adaptation of microbes [1-3].

Four ways that the bacterium, Staphylococcus aureus, can form biofilms. Equal for these biofilms is that they benefit the bacteria, not the host. The bacteria grow in clusters and attach to surfaces to ensure their own survival. S. aureus is the most frequent cause of biofilm associated infections in medical devices and implants.
Biofilms in health and medicine
In the medical field, biofilms are often a concern due to their tendency to form on implants and their resilience to antibiotics. In consequence, biofilms can cause serious illness and failure of surgical procedures and treatments [1-4]. This is mainly due to the EPS and the physiological change of the biofilm-residing microbes as biofilms contain numerous proteins which are not observed in the planktonic and free-living cells [1-3].
Since biofilm-residing bacteria tend to be resilient to the immune system, antibiotics, and other treatments, biofilm infections are typically chronic in nature .
The antibiotic resistant features of the biofilm is a major concern which has fundamental consequences [1, 4]. Antibiotic resistance often occurs because of the persister cells inside the biofilm [1, 5]. In order to survive in the presence of lethal factors, these cells forfeit propagation. That is, when subjected to antimicrobial treatments, the cells change to a state where they do not divide.
Biofilms cause foreign body implant infections
An example of a bacterial strain associated with the infection of implants made from foreign body materials is Pseudomonas aeruginosa , while the most frequent cause of biofilm associated infections in medical devices and implants is Staphylococcus aureus. During an antibiotic treatment, the persisters survive, and once the treatment ceases, they can repopulate. The only solution is thereby to remove the implant [1, 5]. Another problem is that the biofilm-residing cells often slough off and repopulate another place in the body [1-3]. Therefore, biofilms are often studied in research to overcome or avoid the consequences of the formation on medical devices and implants .
The medical importance of biofilm research
Due to the significant human health implications of biofilms, research concerned with biofilm formation, prevention, and treatment can potentially enhance medical and dental practices.
Biofilm has been found to be part of many chronic infections. Therefore, knowledge on how biofilm may contribute to the pathogenesis of disease is important for the development of effective treatments for biofilm associated infections .
The field of research mostly investigates the formation of biofilms on implants and solid support matrices, or scaffolds, to obtain knowledge on the communication between the biofilm cells. Namely, in the hope of finding a solution to the antibiotic resistances or to gain a better understanding of the complex community [2-4].
To reach an understanding of biofilm formation mechanisms, the P3D scaffold can be used to investigate microbe attachment and how biofilm-residing cells interact with 1) each other, 2) synthetic bone implants, and 3) bone cells. Moreover, the lifelike 3D environment can be used to test the effect of antimicrobial treatments.
If you have any questions, please email research@ossiform.com.
References
- Willey, J.M.S.L.M.W.C.J., Biofilms Are Common in Nature, in Prescott’s Microbiology 2017, McGraw-Hill Education, 2 Penn Plaza, New York, NY 10121. p. 151.
- O’Toole, G., H.B. Kaplan, and R. Kolter, Biofilm formation as microbial development. Annu Rev Microbiol, 2000. 54: p. 49-79.
- Tolker-Nielsen, T., Biofilm Development. Microbiol Spectr, 2015. 3(2): p. Mb-0001-2014.
- Del Pozo, J.L., Biofilm-related disease. Expert Rev Anti Infect Ther, 2018. 16(1): p. 51-65.
- Lewis, K., Persister cells and the riddle of biofilm survival. Biochemistry (Mosc), 2005. 70(2): p. 267-74.
- Vestby, Lene K et al. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics (Basel, Switzerland) vol. 9,2 59. 3 Feb. 2020.
- Illustration created with BioRender.com


