Treating mouse skull defects with tricalcium phosphate implants
New research paper: Treating mouse skull defects with 3D-printed fatty acid and tricalcium phosphate implants
The performance of 3D printed bone implants in a mouse model of craniectomy
The performance of P3D Bone as a novel form of individualized ceramic bone implants and controlled-release drug depots has been investigated in previous studies (Jensen et al., 2018; Slots et al., 2017). The results of several laboratory tests and pre-clinical in vivo trials demonstrate that the implants are mechanically strong. Furthermore, they are free from contaminants, support a rapid formation of new vascularized bone, and integrate with neighboring bone in vivo. Furthermore, the fatty acid-based 3D printing method proved that the implants’ compressive strength, resorption speed, and drug release rate can be controlled by varying the fatty acid tail length. Additional pre-clinical data has now been published in the current research paper Treating mouse skull defects with 3D‐printed fatty acid and tricalcium phosphate implants. The study investigates the performance of the 3D-printed bone implants in a mouse model of skull surgery, also known as craniectomy. The aim is to make biodegradable porous implants that can ultimately be fitted to a patient’s anatomy.
 Results
Results
The experiments indicated that the fatty acid-based bioink is very suited to regenerative medicine. This is because it enables rapid and simple 3D printing of biocompatible and bone-forming implants.
In the current in vivo mouse study, Ossiform’s nonpolymeric thermoplastic bioink composed of fatty acids and β-tricalcium phosphate was used to 3D print the skull implants. The bone implants were 3D printed as nets that were subsequently cut into circular implants with nine pores. Some of the implants were sintered to yield pure β-tricalcium phosphate implants. Others were nonsintered to retain the fatty acid, all were sterilized, and some were seeded with stem cells. The results of the study’s two experiments introduced below demonstrated that both the sintered tricalcium phosphate implants and non-sintered fatty acid/tricalcium phosphate implants were highly biocompatible. However, only the sintered implants promoted defect healing, with osseointegration with adjacent bone and the formation of new bone and bone marrow tissue in the implant pores. Stem cells seeded onto these implants engraft and proliferate on the implants after implantation and contribute to bone formation. Meanwhile, the non-sintered implants formed vascularized and cell-rich tissues but no bone. Finally, the implant resorption rate appeared to depend strongly on the fatty acid tail length. Confirming in vivo our previous in vitro observations (Jensen et al., 2018).
The experiments
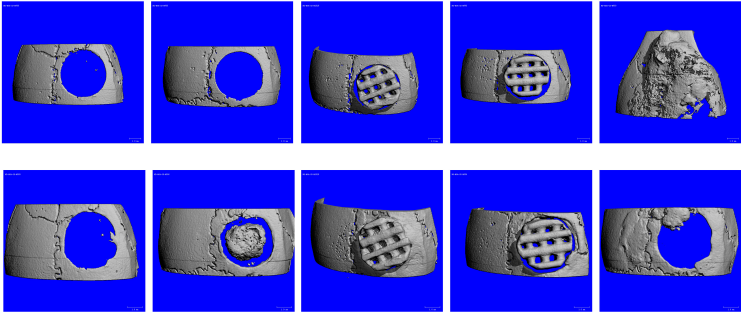
The purpose of the first experiment was to compare the performance of nonsintered lauric acid/tricalcium phosphate implants with sintered implants of pure tricalcium phosphate. The implants were 3D printed using the same technique and implanted in a mouse calvarial defect with and without stem cells. In addition to the nonsintered and sintered implants with and without cells, the experimental groups included a hydroxyapatite-coated collagen sponge with cells which were used as a positive control and a negative control with an untreated empty void.
CT scans along with confirmatory histological images showed that the sintered implants supported bone formation and osseointegration at the implant/skull interface both with and without stem cells (see CT imaging below).
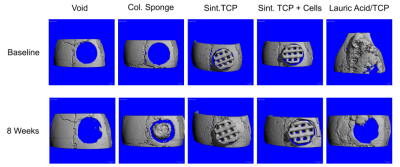
CT imaging of calvarial implants in the first experiment
CT imaging of calvarial implants in the first experiment
The purpose of the second experiment was to determine whether the use of stearic acid instead of lauric acid would be more stable and better promote bone formation in vivo. Furthermore, the mice were tested for immune/foreign body reactions to the implants.
The experimental groups included sintered tricalcium phosphate implants, nonsintered stearic acid/tricalcium phosphate implants, and a clinical grade titanium net as a positive control. While the sintered implants supported the formation of new bone and bone marrow within the defects, the nonsintered stearic acid/tricalcium phosphate implants did not show any signs of new bone development, nor did any of the titanium samples or the nontreated empty void samples. However, the nonsintered implants were full of vascularized cell-rich soft tissue and thus appeared very biocompatible although they did not form bone.
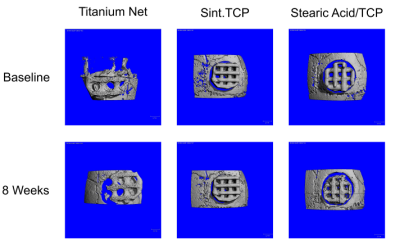
CT imaging of calvarial implants in the second experiment
CT imaging of calvarial implants in the second experiment
The full results is presented in the research article. The results demonstrate that the fatty acid-based 3D printing method is attractive for rapidly and simply creating sintered, biocompatible, and bone-forming β-tricalcium phosphate implants. The implants is aimed for larger patient-specific craniofacial defects and potentially nonsintered fatty acid/tricalcium phosphate implants for soft tissue applications.
Full article and pre-clinical data:
Jensen MB, Slots C, Ditzel N, et al. Treating mouse skull defects with 3D-printed fatty acid and tricalcium phosphate implants. J Tissue Eng Regen Med. 2020; 1–11. Get access to the article here


