Materials for Orthopedic Bone Reconstruction
Orthopedic Implantology
After the cornea, bone is the second most transplanted tissue worldwide. In fact, bone grafts and bone substitute material are used in over four million operations annually (1, 2). Being composed of collagen and phosphate apatite crystals, our bones provide rigidity, strength, and a high degree of elasticity that can alter their shape and structure in response to changes in their environment (3). Naturally, our bones constitute an essential part of the musculoskeletal system that is vital for maintaining our daily activities and our health. However, bone integrity may be compromised by tumors, trauma, infection, metabolic diseases, congenital bone defects, and more.
Orthopedic implantology aims to correct issues arising in the human musculoskeletal system. The course of degeneration of musculoskeletal tissues presents itself as a topic of exhaustive research since the prevention of bone- and joint diseases is a desired hallmark of orthopedics. However, in the present era of the medical field, no effective treatments exist for musculoskeletal disorders, such as osteoarthritis and progressive spinal deformity (4).
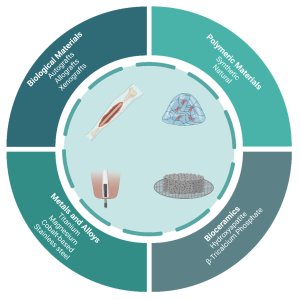
Figure 1: Overview of the broad classifications associated with orthopedic implant materials
Biomaterials in the Spotlight
Biomaterials have been used for the treatment of human diseases as early as 2000 BC. During these ancient times, the Egyptians utilized ivory to replace lost teeth, and employed braces and splints to support and protect fractured bones after surgical procedures (5). Traditionally, allograft bone substitutes have been a mainstay in the reconstruction of bone defects in most parts of the world. Although a viable alternative to autograft bone, allograft bone raises concerns of potential disease transmission, recipient rejection, high cost, and limited availability (6). Furthermore, in some countries like Japan, allogenic bone is difficult to obtain due to socio-religious concerns (7).
During the 1950s, the main goal of the first generation of biomaterials was that they should be bioinert. Then, in the 1980s, the trajectory changed for the second generation of biomaterials to be bioactive, and since the 2000s, the third generation of biomaterials had aimed to regenerate functional tissues (8).
Today, research on bone regeneration has turned into a very promising field, with the use of resorbable biomaterials in combination with living cells. Biomaterials function as physical support to promote cell growth and to simultaneously avoid spillover and asymmetric distribution of cells. This is especially important in inducing the synthesis of cartilage extra-cellular matrix (ECM) and regeneration of functional tissues. More importantly, biomaterials must be biocompatible and exhibit cell adhesive surface properties with suitable microstructures to induce three-dimensional cell proliferation. Finally, modern biomaterials must also display strong mechanical properties to support the newly regenerated tissue in vivo (4). Biomaterials may be categorized into four broad classes: biological materials, metals and alloys, polymeric materials, and bioceramics, which are presented in Figure 1.
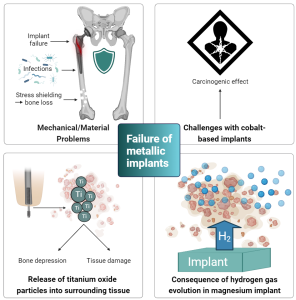
Figure 2: Overview of current challenges with metal-based orthopedic implants.
Metals and Alloys
Metals and their alloys are widely used for repairing and/or replacing damaged bone tissue due to their high mechanical strength and fracture toughness, making them more favorable for load-bearing applications compared to bioceramic- and polymeric materials. In fact, non-biodegradable metals such as titanium and its alloys, stainless steel, magnesium, and cobalt-based alloys have been amongst the most widely used biomaterials for manufacturing medical implant devices. Especially, Titanium (Ti) and its alloy Ti6Al4V are popular ingredients for developing lightweight bone reconstruction devices with excellent mechanical properties, corrosion resistance, and great biocompatibility. While titanium is generally considered to be the metal with the best biological safety, cobalt-based alloys and stainless steel have also been used to manufacture a wide range of permanent (i.e., non-resorbable) medical devices, including hip, shoulder, and knee implants (9, 10)
Generally, most metallic components dissolve chemically and/or electrochemically in the human body. Therefore, these biomaterials are prone to corrode in such environments, which may lead to inflammation and implant loosening (11, 12). Upon exposure to air, titanium surfaces develop an oxide layer that provides the implant with fortified corrosion resistance. However, as this oxide layer is destroyed, Ti particles are released from the implant and distributed in the surrounding tissue. These particles may be harmful but are difficult to eliminate from the body altogether. For reference, in a healthy human body, the titanium content should not exceed 15 mg per 70 kg body weight (10).
Whether titanium, cobalt chrome, or stainless steel, once these metals are implanted into bone, most of the physiological loading is transferred to the implant, distant from the comparatively more compliant bone. Consequently, the altered load transfer in the native bone leads to the bone becoming under-loaded compared to its innate state. Therefore, bone, a living tissue that is highly sensitive to mechanotransduction, resorbs, and loses mass by the adaptive process known as bone remodeling. This phenomenon is appropriately termed “stress shielding” and reduces the quality of the remaining bone stock leading to a significantly increased risk of developing fractures and aseptic loosening of the implant. Furthermore, such traditional metal implants are often associated with post-operative infections.
Aside from inducing stress shielding, cobalt-based alloys have been categorized as being carcinogenic in nature. In fact, a recent regulatory hazard classification review by the European Chemicals Agency (ECHA) described metallic cobalt as a Class 1B Carcinogen (presumed to be a cause of cancer in humans) (13).
Meanwhile, magnesium has emerged as a third generation osteoconductive biomaterial for regenerating and supporting bone tissue. Unlike stainless steel, titanium, and cobalt-based metal, magnesium is biodegradable and possesses anti-bacterial properties. Furthermore, it has a lower risk of stress shielding as its elastic modulus is closer to human bone (9). However, its use is limited by its rapid corrosion once implanted, leading to implant deterioration before the healing of the bone is complete. Moreover, despite its proven suitable biocompatibility with bone, the innate nature of magnesium degradation is associated with the formation of hydrogen gas, which forms gas cavities in the surrounding implantation tissue. Excessive hydrogen gas development creates pressure that induces numerous mechanical disturbances to bone regeneration, resulting in distinct callus formation.
Polymeric Materials
Various biomaterials are utilized to fabricate implants that may overcome the challenges associated with metal implants. These include synthetic polymers which are increasingly used as the main material component or part of a composite material. Synthetic polymers demonstrate reproducible and predictable physiochemical, mechanical, and biodegradable properties, all of which can be proximately tuned in response to the environment.
Poly(a-hydroxy esters) (PHEs) are a class of synthetic, biodegradable polymers that have been extensively exploited for the preparation of scaffolds for tissue engineering. These synthetic biodegradable polymers are often used as a blend of each other or combined with natural polymers or ceramics to promote biological integration (14, 15). The PHEs include poly(lactic acid) (PLA), poly(glycolic/lactic-co-glycolic acid) (PGA/PLGA) and poly(e-caprolactone) (PCL). Although all PHE polymers have received FDA approval for various biomedical applications, they are limited by substantial drawbacks that impede their in vivo and in vitro applications. One of the considerable drawbacks of PHEs is their hydrophobicity, which can adversely affect pivotal cellular properties, such as phenotype, growth, and proliferation. Thus, their in vivo application requires further processing to increase biocompatibility. Furthermore, acidic by-products of the polymer degradation can cause severe adverse tissue reactions, halter cell proliferation and new bone formation, and lead to early implant deterioration. Thus, despite their easy manufacture and large availability, synthetic polymeric materials are only ideal when optimally combined with natural matrices or ceramics that can facilitate their biological integration (4, 16).
Image 1: β-Tricalcium Phosphate powder
Bioceramic Bone Graft Substitutes
Characterized by their osteogenic, osteoconductive, and osteoinductive properties, bioceramics have been universally employed in the field of orthopedic implantology and traumatology as bone graft substitutes. Bioceramics can broadly be divided into three categories: (I) nearly inert (e.g., zirconia), (II) bioactive (e.g., bioactive glass), and (III) resorbable (e.g., calcium phosphates), where the former is rarely chosen for regenerative medicine due to its lack of tissue regeneration abilities (17, 18). Other bioceramics possess several properties that are highly beneficial for bone reconstruction. These include biocompatibility and bioactivity, which ensure reduced risk of adverse reactions and inflammation, improved tissue regeneration, and enhanced osteointegration (19). The most widely studied material for the production of ceramics is calcium phosphate, particularly hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) due to their similarity to natural bone.
Bone is made from an apatitic calcium phosphate mineral, similar to that of HA. However, HA is more crystalline than bone and β-TCP, making it less degradable and increasing the risk of deformities and fractures upon implantation. Conversely, β-TCP has a solubility similar to that of natural bone mineral and is therefore resorbable and replaced by new bone through resorption by osteoclasts upon implantation. In fact, β-TCP has been proven to have superior properties compared to HA in terms of cell-mediated resorption and osteoconductivity several times, with the former exhibiting a more appropriate degradation rate which is highly beneficial to the field of orthopedic surgery (20). This osteotransductive potential of β-TCP makes it an attractive material for bone substitutes (21).
Recently, the 3D printing of bioceramic materials has surfaced and planted its roots in a wide range of research and pre-clinical applications. By enabling total controllability of the materials’ size and shape, the durability may be increased and the applicability of ceramics is extended beyond the traditional uses of ceramics in the form of pastes, granules, and molded blocks; The porous, degradable bioceramics are intentionally designed to be incorporated into bone as natural replacements at void-filling and load-bearing sites and often used in combination with other biomaterials, including natural grafts, metals and polymers (22).
So, which material to choose?
Historically, various routes have been proposed for obtaining the ideal material for optimizing bone reconstruction. However, no ideal material or technique has been attained to endow a designable biomaterial with the necessary properties to completely control bone reconstruction configuration. To accomplish this, further research is warranted to explore the effects of modified biomaterials, perhaps with β-TCP as the leading candidate.
Nevertheless, in a near future where cell- and molecular biology, material science, and orthopedic implantology are effectively merged, chances are that the new generation of biomaterials will be able to reach clinics with exceptional and sustainable regenerative approaches to ultimately advance the field of bone reconstruction.
Learn more about Ossiform’s resorbable bone graft substitute, P3D Bone.
References
- Turnbull, G. et al. (2018). 3D bioactive composite scaffolds for bone tissue engineering.
- Ghezzi CE, Rnjak-Kovacina J, Kaplan DL. (2015) Corneal tissue engineering: recent advances and future perspectives.
- Freemont AJ. (1993). Basic bone cell biology.
- Li, Qing & Mai, Y.-W. (2017). Biomaterials for Implants and Scaffolds.
- Al-Shalawi FD, et al. (2023). Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers.
- Yaszemski, M.J. (2003). Biomaterials in Orthopedics (2nd ed.). CRC Press.
- Yamamoto N, Hayashi K, Tsuchiya H. (2019). Progress in biological reconstruction and enhanced bone revitalization for bone defects.
- Holban A. and Grumezescu A.M. (2019). Materials for Biomedical Engineering. Hydrogels and Polymer-Based Scaffolds.
- Uppal G, et al. (2021) Magnesium based implants for functional bone tissue regeneration – A review.
- Zhou Z, et al. (2021) The unfavorable role of titanium particles released from dental implants. Nanotheranostics.
- Li, H., Khor, K. A., and Cheang, P. (2003). Impact Formation and Microstructure Characterization of thermal Sprayed Hydroxyapatite/titania Composite Coatings.
- Manam, N. S. et al. (2017). Study of Corrosion in Biocompatible Metals for Implants: A Review.
- https://echa.europa.eu/da/substance-information/-/substanceinfo/100.028.325.
- Nooeaid P, Salih V, Beier JP, Boccaccini AR (2012) Osteochondral tissue engineering: scaffolds, stem cells and applications.
- Ondrésik M, et al. (2017) Management of knee osteoarthritis. Current status and future trends.
- Oliveira J. M., et al. (2018). Osteochondral Tissue Engineering Challenges, Current Strategies, and Technological Advances: Challenges, Current Strategies, and Technological Advances.
- Maia F. R. et al. (2019). Chapter 32 – Natural Origin Materials for Bone Tissue Engineering: Properties, Processing, and Performance. Pages 535-558.
- Min Wang, Lin Guo, Haoran Sun. (2019). Manufacture of Biomaterials. Pages 116-134.
- Vaiani L, et al. (2023). Ceramic Materials for Biomedical Applications: An Overview on Properties and Fabrication Processes.
- Lu, Haiping & Zhou, et al. (2021). Current Application of Beta-Tricalcium Phosphate in Bone Repair and Its Mechanism to Regulate Osteogenesis.
- Marc Bohner, Bastien Le Gars Santoni, Nicola Döbelin. (2020). β-tricalcium phosphate for bone substitution: Synthesis and properties.
- Block JE, Thorn MR. (2000) Clinical indications of calcium-phosphate biomaterials and related composites for orthopedic procedures.



