3D printed resorbable implants in the mandible of pigs
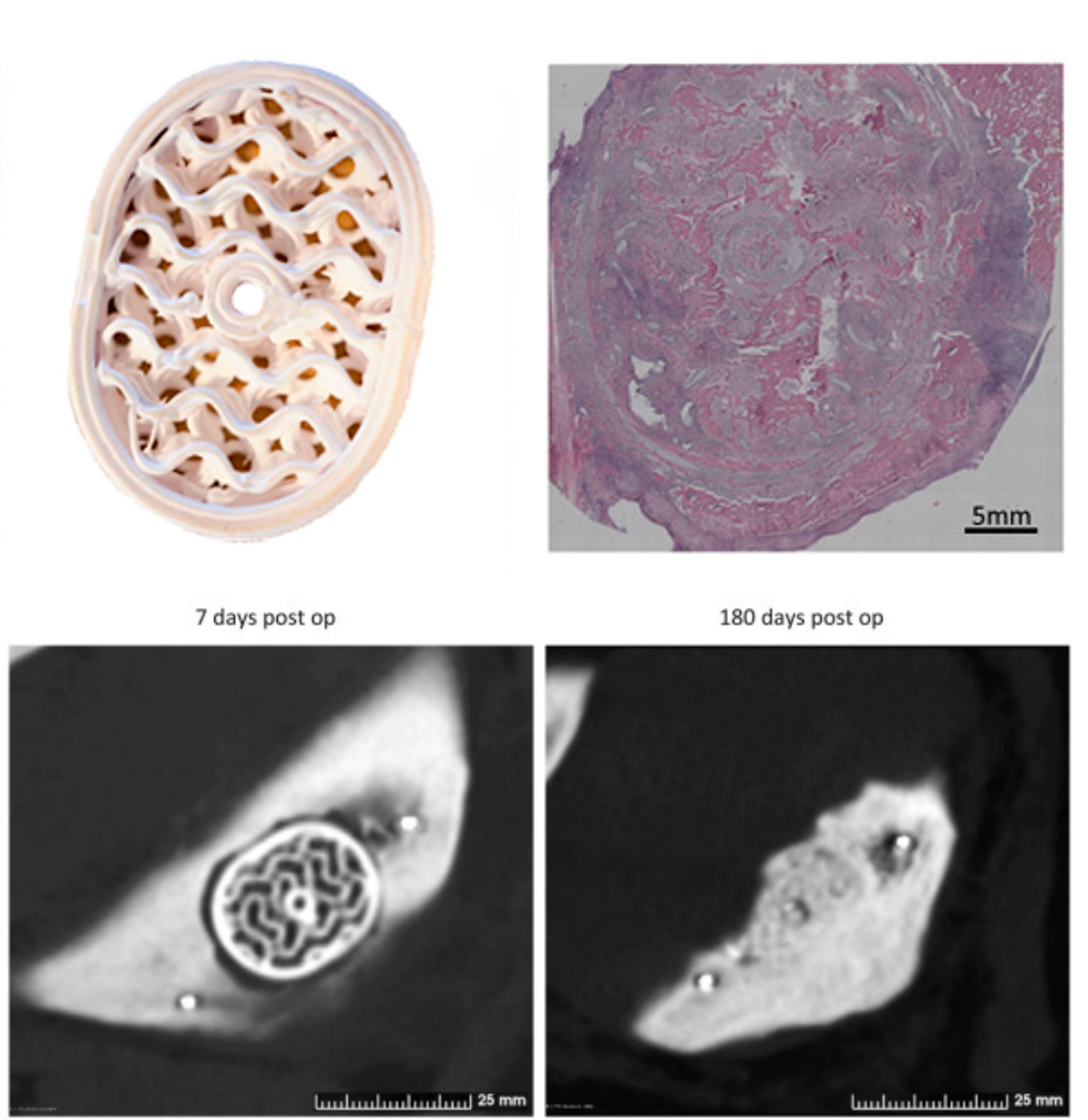
The performance of 3D printed resorbable implants in the mandible of pigs
New paper: Comparison of off-the-shelf b-tricalcium phosphate implants with novel resorbable 3D printed implants in mandible ramus of pigs
3D printed patient specific implants derived from CAD/CAM-based technologies are presented as an alternative to preformed bone graft substitutes. Yet, today, clinicians must still choose between patient specificity or implant properties that are favorable for bone remodeling. This is leading to off-the-shelf solutions such as the β-tricalcium phosphate granules and preformed blocks that are used today.
Ideal patient-specific Implants not only exhibit exact fitting, porosity, density, and volume. They are also insoluble, osteoconductive, osteoinductive, have resorption properties that resemble native bone, and allow ingrowth and formation of new bone tissue. These are properties that, in combination, are not found in commercially available patient-specific implants (Thygesen et al, 2022).
The resorbable properties of P3D Bone have previously been investigated in several studies (Jensen 2020, Jensen 2018, Slots 2017). In this pilot study, the 3D printed P3D Bone has been directly compared to a commercially available pre-formed β-tricalcium phosphate implant in relation to defects in the mandible ramus of pigs. The study aimed to analyze the performance of the implants that fulfill the aforementioned requirements in a clinically relevant implantation model. This is where the implant, defect, and bone are similar in size to what would be expected in human patients.
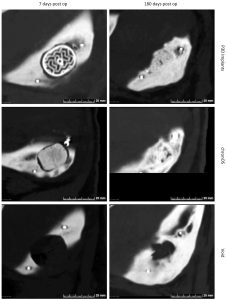
Figure 1: CT-scans representative of voids and implant sites 7- and 180-days post op.
The results of the study showed that the commercially available off-the-shelf implant and the P3D Bone performed equally well, with predicted osteointegration medially and laterally and minimal gapping between the implants and native bone.
The study
The purpose of the study was to directly compare the P3D Bone implant with an off-the-shelf implant made from β-tricalcium phosphate. Empty voids were used as controls to showcase the natural ability of the pigs to mend a defect of that size.
Eight pigs were anesthetized, and a predetermined hole was dissected in the mandible on each side of the pig. Hereafter the P3D Bone implant was inserted on one side. On the other side either the control implant was inserted, or the hole was left as a void. The pigs were stabled individually for six months before evaluating the performance of the implants by CT-scanning and histology of jaw sections.
CT findings
Six months after the surgical procedure, the pigs were CT-scanned. The analysis of the CT images showed promising results regarding integration and ossification around the P3D Bone and control implants as well as the resorption of implants.
The analysis showed various degrees of integration, resorption, and ossification of the voids. Furthermore, bone integration and ossification at the perimeters and middle of the P3D Bone implant resembled natural bone presenting with a cortical surface and spongious-like bone within the implants.
Histological analysis

Figure 2: Histological images of the P3D implant perimeter (A) and core (B–C) and of the off-the-shelf implant cores (D) and the resected non-treated voids (E–F) after 6 months.
The findings from the CT-scan were supported by the histological analysis. They showed that most of the P3D Bone implant was resorbed leaving only a few small pieces of the original implant. As β-tricalcium phosphate does not degrade on its own inside the body, the disappearance of the implants after six months was likely due to resorption by osteoclasts.
The histology slides also revealed that the inside of the P3D Bone implants was filled with soft tissue, muscle tissue, and bone tissue. The bone tissue was dense with small canals of soft tissue containing blood vessels. The comparison of the P3D Bone implant site with native bone suggested that the newly formed bone was mature. Meanwhile, the negative control voids were filled with adipose tissue, fibrous/connective tissue, and muscle tissue with no bone tissue.
Concluding remarks
The study showed that the P3D Bone implants performed at least equally as well as the commercially available off-the-shelf implants. It also showed that newly formed mature bone was integrated within the implants after three and six months. Furthermore, the study concluded that the P3D Bone implants are highly biocompatible and support natural cellular and systemic responses.


